Abstract
INTRODUCTIONHairy cell leukemia (HCL) is a rare B-cell malignancy where there is a need for treatment options for patients who do not benefit from purine nucleoside analogs (PNA) due to short remission duration or other conditions making them unsuitable to receive them. Ibrutinib is an oral inhibitor of Bruton Tyrosine Kinase (BTK) that is effective in a variety of B-cell malignancies and tolerable for indefinite extended administration. As inhibitors of BTK have been successful in other B-cell cancers it was a rational therapeutic approach in HCL. We conducted a phase 2 study of ibrutinib in both classic and variant HCL and have previously reported outcomes for the first 37 patients treated. We now report the primary endpoint for the entire cohort (n=44) as well as extended follow up.
METHODS This phase 2 multisite study is funded by the National Cancer Institute (NCI) with ibrutinib supplied by Pharmacyclics. The protocol was IRB approved at all participating sites. Adults (≥18 years old) with both classic and variant HCL were eligible if they met criteria for requiring treatment. They had to have received prior treatment with a PNA or be unsuitable to receive one. Patients with the HCL variant could have no prior treatments. Patients were initially treated with ibrutinib at either 840mg daily or 420mg daily. Based on concerns for toxicity 420mg was selected as the dose for more recently enrolled patients and those started at 840mg were allowed to reduce to 420mg. Response was assessed at 32 and 48 weeks after starting treatment as well as any time after that at the discretion of the investigator. The study was open to accrual in April 2013 and completed enrollment in March 2021. Data reported include follow up as of May 25, 2022. Response rates were calculated for each time point and were compared by HCL subtype with Fisher's exact tests. Kaplan Meier methods were used to assess progression-free (PFS) and overall survival (OS), with log-rank tests used to compare the outcomes by subtype.
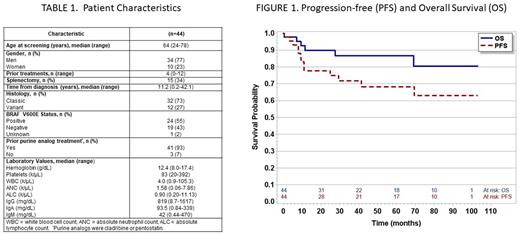
RESULTS A total of 44 patients were included with characteristics in Table 1. The median follow up is 3 years (range 2 weeks to 8.6 years) and 16 (36%) patients remain on treatment. There were 13 patients initially treated with 840mg and 31 with 420mg. All except 1 patient continued treatment with 420mg. Reasons for discontinuing treatment were progressive disease (n=12), adverse events (n=9), elective withdrawal (n=5), and death (n=2). There were 6 deaths in the study, 2 during treatment from pneumonia, 2 from progressive disease, and 2 from infections after discontinuing ibrutinib.
The primary endpoint was overall response at 32 weeks and was 29% (12/42, 2 missed due to COVID-19) and improved to 41% (18/44) at 48 weeks and 59% (26/44) at any time. 7 (16%) achieved a best response of complete remission and 19 (43%) partial remission. Response rate was not different between patients with classic or the variant subtype. The median PFS and OS were not reached (Figure 1). The estimated 60-month PFS and OS were 68% (95% CI: 50%-81%) and 87% (95% CI: 71%-94%) months, respectively. There were no significant differences in PFS and OS between classic and variant subtypes.
The most frequent adverse events (AEs) regardless of attribution to study treatment were diarrhea (59%), fatigue (52%), myalgias (52%), nausea (48%), thrombocytopenia (45%), upper respiratory infection (45%), bruising (41%), cough (41%), headache (41%), and hypertension (41%) which are all established AEs observed with ibrutinib in other diseases. The most frequent grade ≥3 AEs were hematologic with thrombocytopenia (23%), leukopenia (23%), lymphopenia (20%), neutropenia (18%) with grade ≥3 lung infection (16%) and hypertension (11%) also noted.
CONCLUSIONS With a median follow-up length of 3 years, patients with HCL continue to benefit from ibrutinib with an estimated 60-month PFS of 68%. In this evaluation of 44 patients the rate of response to ibrutinib at any time was 59% which is similar to 54% in the initial report with 37 patients (Rogers et al., Blood 2021). The durable PFS in this difficult to treat population makes ibrutinib an effective therapy for select patients with HCL who are not expected to benefit from a PNA.
Disclosures
Rogers:Innate Pharma: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy, Other: Travel Funding; Novartis: Research Funding; Janssen: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Blachly:MingSight Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; INNATE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kreitman:NIH: Patents & Royalties: Co-Inventor for Moxetumomab Pasudotox Patent; AstraZeneca: Other: Clinical Testing, Research Funding; Pfizer: Other: Receive drugs for clinical testing; Teva: Other: Receive drugs for clinical testing; Genentech: Other: Drugs for clinical testing, Research Funding; Novartis: Other: Drugs for Clinical testing, Research Funding. Ravandi:Xencor: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding; Amgen: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Prelude: Research Funding; AstraZeneca: Consultancy; Syos: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding. Grever:Ascerta: Consultancy; Innate Pharma: Consultancy; Hairy Cell Leukemia Foundation: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy; Pharmacyclics: Consultancy; Serono: Consultancy; Axio: Consultancy.
OffLabel Disclosure:
This study uses ibrutinib to treat hairy cell leukemia which is not a label indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal